Cancer Treatment Remission Statistics
Detailed statistical data on long term cancer remission with use of the Issels Immuno-Oncology program.
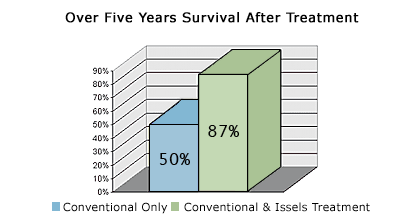
Statistical Data of Long Term Cancer Remission for Patients Five Years after the Issels Treatment
Cancer medicine as practiced at our Issels Integrative Immuno-Oncology Centers evolved from the comprehensive treatment system developed by Josef M. Issels, M.D., the world-renowned pioneer of integrative cancer medicine.
Josef M. Issels, M.D. reported in "Immunotherapy in Progressive Metastatic Cancer, A Fifteen Year Survival Follow-up" Clinical Trials Journal (London) 1970. 7, NO. 3, A Peer Review Paper, pp. 357-366, that out of 370 patients with various cancers who were given the Issels Immunotherapy, shortly after surgery or irradiation, 322 (87%) were alive and well after five years without relapse or detectable metastases.
A restoration of the immune functions can reduce the incidence of relapse from about 50% (worldwide statistic) to 13% by combining conventional treatments and integrative immunotherapy for cancer.
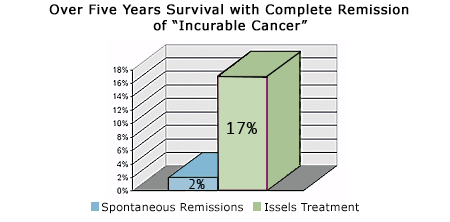
John Anderson, M.D., Teaching Professor at King's College Hospital, London, reported 17% remission rate of histologically verified metastatic malignomas by the Issels Immunotherapy for Cancer. The independent randomized study comprised 570 patients with various cancers after all conventional methods were exhausted (General Practitioner, March 1971, pp. 15-16, London).
An independent statistical study by A.G. Audier, M.D., University of Leiden, Holland "Immunotherapie Metastasierender Malignome" Die Medizinische 1959, NO. 40, A Peer Review Paper, pp. 1860-64, reported a 16.6% remission rate of histologically verified metastatic malignomas by the Issels Immunotherapy for Cancer. The study comprised 252 patients with various cancers after all conventional methods were exhausted (worldwide cure rate is 2%).
Issels In-House Research Studies
The paper "Complete Remission of Innumerable Lung Metastases from Melanoma through the Issels Immunotherapy Protocol" describes the following case.
Within 4 weeks of the non-toxic Issels Integrative Immunotherapy program, complete remission of uncountable lung metastases of melanoma was achieved as revealed by a CT scan and reconfirmed by a control CT scan after 4 months.
Image 1. Pre Therapy CT scan of Chest:
Innumerable nodules are seen in bilateral lung fields consistent with metastatic melanoma.
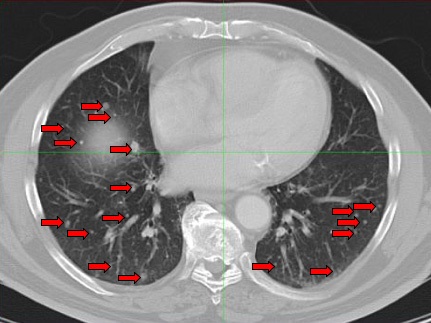
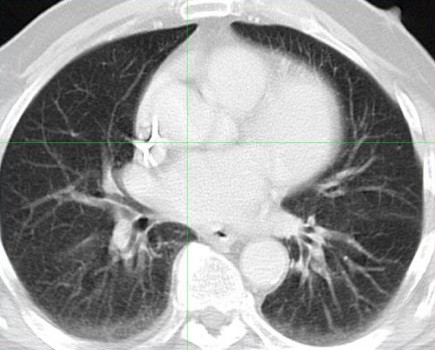
Image 2. Post-therapy CT scan of chest performed 4 weeks after treatment.
Images reveal resolution of all previously seen lung nodules suggesting complete response to treatment.
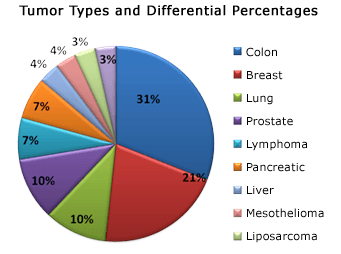
Another study, Natural Killer Cell Activation through the Issels Immunotherapy Protocol, included 129 cancer patients with pathologic confirmation of disease who underwent the Issels Immunotherapy for Cancer program at the Issels Medical Center in Santa Barbara, California.
The Natural Killer Cell, NKC, response to the Issels Immunotherapy protocol revealed an average 48% increase in absolute Natural Killer Cell levels per patient in approximately 3 weeks.
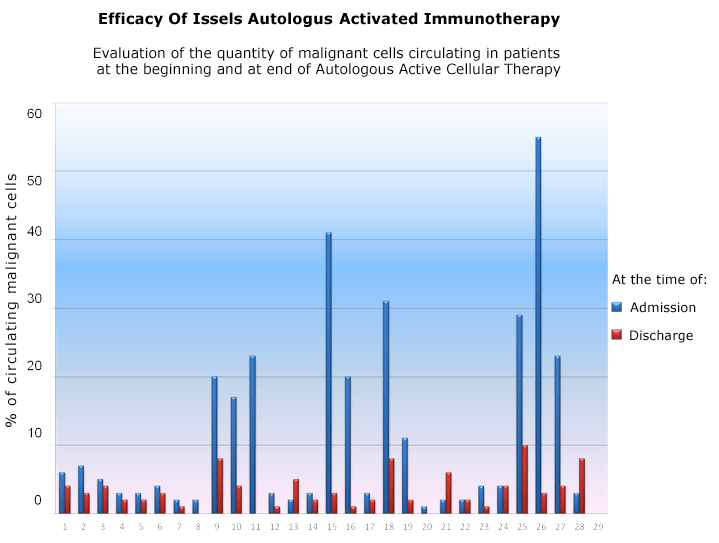
Immunohematologist Alejandra Calderon, M.D. conducted a study about the Efficacy of the Autologous Active Cellular Immunotherapy of the Issels Program, which revealed 86.2% reduction of the circulating cancer cells by the end of the initial intensive treatment.
Efficacy of Issels Autologous Active Cellular Immunotherapy
Statistical Analysis of Treatment Data Demonstrated an Overall Significant Improvement in Circulating Malignant Cells
The Department of Epidemiology, Mailman School of Public Health, Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons at Columbia University, New York did a best case study at the Hufeland clinic of Dr. Josef Issels' successor in Germany, Dr. Wolfgang Woeppel, M.D. (08.15.1946 ?07.10.2006). The abstract of the publication is quoted below.
Integr Cancer Ther. 2005 Jun;4(2):156-67. [Link to the Publication.]
Cancer Outcomes at the Hufeland (Complementary/Alternative Medicine) Klinik: A Best-Case Series Review
Jacobson JS, Grann VR, Gnatt MA, Hibshoosh H, Austin JH, Millar WS, Neugut AI. Department of Epidemiology, Mailman School of Public Health, Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons.
PURPOSE: A best-case series review is an efficient tool with which to screen complex complementary and alternative treatments for cancer as candidates for further study.
STUDY DESIGN: The National Cancer Institute and other agencies have adopted the best-case series method to evaluate cancer treatments involving complementary and alternative medicine (CAM) for further study. The authors conducted a best-case series review of the Hufeland Klinik.
Established in 1985 in Bad Mergentheim, Germany, this facility treats more than 500 cancer patients per year. Hufeland treatment includes dietary modification, injections, ozone therapy, active fever therapy, psychotherapy, and sometimes hormone therapy and/or low-dose chemotherapy. The goal of the treatment is to prolong survival and to maintain good quality of life.
METHODS: The clinic provided summaries of 27 cases in which patients with longer than expected survival had agreed to make their medical records available for review. The review involved pathologic confirmation of disease and radiologic confirmation of complete response (CR) or partial response (PR) not attributable to conventional treatment.
RESULTS: Based on the summaries and an exhaustive 2-year search for medical records, slides, and imaging data, 12 of 27 cases were selected for full review, and 5 (3 CRs and 2 PRs) were judged best cases.
CONCLUSION: Most patients with common cancers receive conventional treatment before coming to Hufeland, and many are treated with chemotherapy and/or hormonal therapy while there. Hence, only a few could be considered for review. With 5 of 12 patients showing a treatment response, the authors conclude that the Hufeland treatment merits further study. They also recommend the development of criteria with which to evaluate best-case series reviews of complex CAM treatments for patients with advanced cancer.
PMID: 15911928 [PubMed abstract]